Answer:
Option B,D
Explanation:
For the elements with atomic number (Z) larger than 20,
Neutrons (n) > Protons (p); Thus, n/p>1. Thus , there is upward deviation from linearity
If n<p, thus n/p <1, then
(a) By $\beta^{-}$ - decay , $_{0}^{1}n\rightarrow ^{1}_{1}p+$ $_{-1}^{0}e$
neutron changes to proton. Thus (n/p) ratio further decreases below 1.
Thus, this decay is not allowed
(b)By orbital or K - electron capture.
$^{1}_{1}p+$ $_{-1}^{0}e\rightarrow _0^1n^{}$ proton changes to neutron, hence , (n/p) ratio increases.
Thus stability increases. Thus correct.
(c) Neutron emission further decreases n/p ratio.
(d) By $\beta^{+}$ - emission . $^{1}_{1}p\rightarrow _0^1n^{}+$ $_{+1}^{0}e$ proton changes to neutron.
Hence,n/p ratio increases, Thus correct
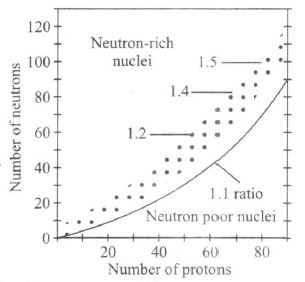
Plot of the number of neutrons against the number of protons in stable nuclei (shown by dots)